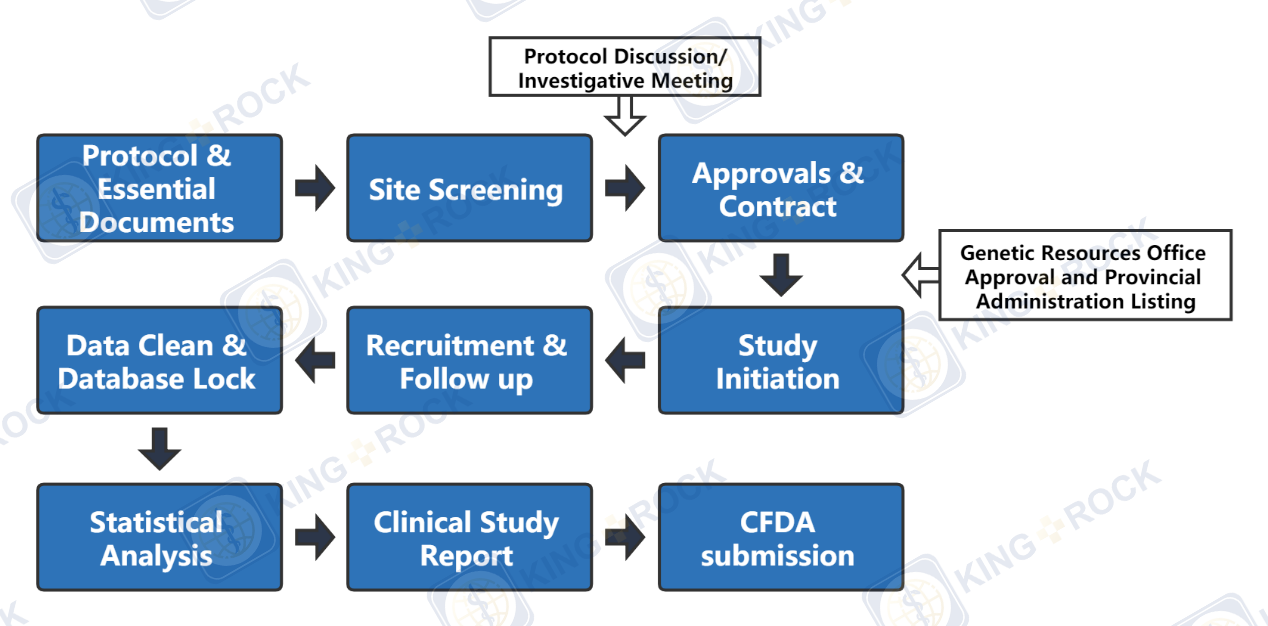
Clinical research is very important to the successful development of medical devices. Through clinical trials, the safety and effectiveness of medical devices can be reasonably evaluated, which provides scientific basis for medical devices launch; on the other hand, clinical trials are time-consuming and costly. It may increase social costs and bring heavy economic burden to manufacturers and even consumers If not properly managed, thus affecting the development of the medical device industry.
Our services cover the entire lifecycle of medical devices, and we can be in-depth cooperation from the product development stage and animal study stage. With accumulation on the understanding of your products from the very beginning, we can better communicate with investigators during the clinical trial, give professional advice, and comprehensively control the quality of the trial.
For clinical research, KINGROCK provide comprehensive professional services from strategy development, protocol design, project operation, data management and analysis to registration. This will optimize project management operations, raising efficiency and minimizing risks during trials operation.


Key operational team members of KINGROCK originate from well-known CRO companies and medical device companies and the abundant experience in each stage of clinical trials and observational studies will help to provide customers with comprehensive professional services.
KINGROCK accumulated rich experience during feasibility study to post-marketing trials on Oncology, Cardiovascular, Ophthalmic, Dermatology, Stomatology, Wound Care, and Infective diseases to mention just a few. We also maintain excellent cooperative relationships with key investigational sites and investigators and we’ve also maintained good relations with NMPA, CMDE and other regulatory supervision departments.


We provide cross-functional and cross-departmental cooperation to help your products be commercialized at full speed and in the most efficient manner.
Our primary goal is in coincidence with yours, to accelerate your products to market, help you achieve greater return on investment, and most importantly, bring better medical device products to life.