 Lab software & hardware: Our lab is established according to the international standards and GLP quality system requirements, and also equipped with world-leading DSA operating room and surgical equipment, which can meet the preclinical experimental needs of various kinds of implantation and interventional surgery
Lab software & hardware: Our lab is established according to the international standards and GLP quality system requirements, and also equipped with world-leading DSA operating room and surgical equipment, which can meet the preclinical experimental needs of various kinds of implantation and interventional surgery
 Safety and Performance: comprehensive evaluation of functionality, safety and operability
Safety and Performance: comprehensive evaluation of functionality, safety and operability
 Histopathology: qualitative and quantitative assessments
Histopathology: qualitative and quantitative assessments
 Surgeon Training: comprehensive clean operation rooms, on-site observation rooms for operations, and remote transmission observation equipment, modern multimedia conference room, on-site support and guidance from qualified laboratory team
Surgeon Training: comprehensive clean operation rooms, on-site observation rooms for operations, and remote transmission observation equipment, modern multimedia conference room, on-site support and guidance from qualified laboratory team
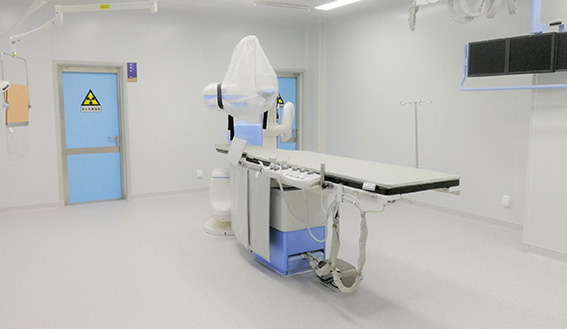
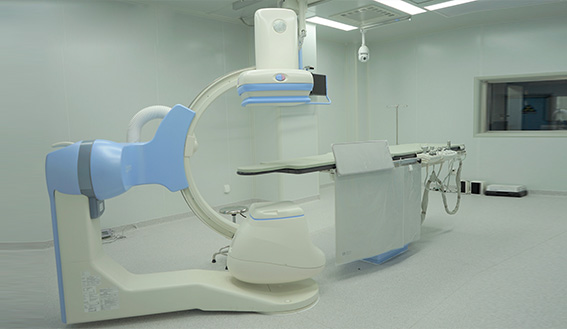
Customized efficacy and performance and other testing:
 Coronary artery and peripheral vascular stent, PTCA catheter, heart valve (aortic valve replacement)
Coronary artery and peripheral vascular stent, PTCA catheter, heart valve (aortic valve replacement)
 Patch, orthopedic / hearing implant device, intraocular lens (IOL), biodegradable surgical nets, biodegradable polymer films, degradable surgical sutures
Patch, orthopedic / hearing implant device, intraocular lens (IOL), biodegradable surgical nets, biodegradable polymer films, degradable surgical sutures
 Extracorporeal circulation system and catheter, blood component separator, hemodialysis catheter and dialysate, angiography bag and solution, peritoneal dialysis box, hemostatic material, blood bag
Extracorporeal circulation system and catheter, blood component separator, hemodialysis catheter and dialysate, angiography bag and solution, peritoneal dialysis box, hemostatic material, blood bag
 Decellularized devices (SIS related products, artificial cornea, nerve repair material, cartilage repair material, urethral repair material, etc.), biological amniotic membrane and some collagen devices (collagen suture and patch)
Decellularized devices (SIS related products, artificial cornea, nerve repair material, cartilage repair material, urethral repair material, etc.), biological amniotic membrane and some collagen devices (collagen suture and patch)
 Endoscope, tracheostomy tube, wound dressing, ophthalmic instrument
Endoscope, tracheostomy tube, wound dressing, ophthalmic instrument






